AUSTIN, TX February 7, 2013 – Wenzel Spine, Inc., a medical device company focused on providing minimally invasive, stand-alone alternatives to traditional spinal fusion, today announced they have received 510K clearance to market the Zero-Profile Stand-Alone VariLift Cervical (VariLift-C) Expandable Interbody Fusion System.
VariLift-C is indicated for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one disc level. VariLift-C is used to facilitate intervertebral body fusion in the cervical spine and is placed in a unilateral or a bilateral fashion via an anterior approach at the C3 to C7 disc levels using autograft bone.
VariLift-C may be used with or without supplemental fixation.
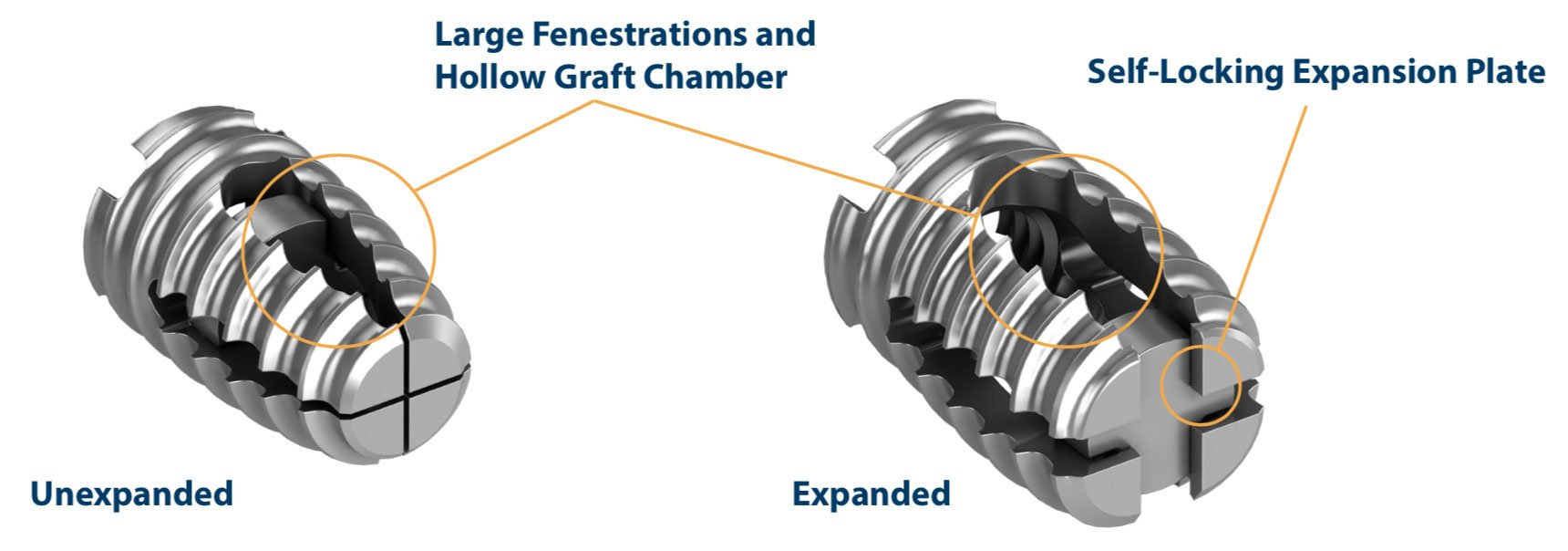
Chad Neely, the Chief Executive Officer of Wenzel Spine, said, “We are pleased that the FDA granted clearance to market VariLift-C. This will be the only expandable cervical interbody fusion device available which may be used without anterior plating, integrated screws or other types of supplemental fixation. VariLift-C represents a true Zero-Profile, Stand-Alone options for surgeons to use in ACDF cases.”
Mr. Neely added, “VariLift-C is a proven stand-alone solution which is essential for today’s surgeons to simply ACDF procedures, improve clinical outcomes for patients, and reduce overall cost to the healthcare system. Wenzel Spine and VariLift are in the unique position to be able to offer stand-alone expandable solutions for the entire segmented spine via ACDF, PLIF, TLIF and ALIF approaches.”
Wenzel Spine’s Cervical VariLift® Stand-Alone Expandable Interbody Fusion System is scheduled to become commercially available in the US and Europe in April of 2013.
